The FDA and Drugs for Cancer
By Marcus M. Reidenberg, MD, FACP
Weill Cornell CERT
What is needed for a drug to be approved for marketing by the FDA? It must be found to be effective by well controlled studies and safe for its intended use. This leaves open the specifics about criteria for determining if a drug is effective and if it is safe for its intended use.
Customarily, two criteria are used to evaluate effectiveness for cancer drugs. One is length of life after start of treatment. The other is duration of progression-free survival. Progression-free survival is the time it takes from the start of treatment to the time when the cancer appears bigger on imaging. Traditionally, progression-free survival was thought to predict a longer life. Recent drug trials in breast cancer found that it did not (1-3). This is the cause of the present conflict about Avastin in breast cancer.
The patients given Avastin plus chemotherapy had longer time until progression than those given chemotherapy alone. This led to an “accelerated approval” by the FDA. Accelerated approval was developed to speed effective drugs to market and use. It is based on short-term end points likely to predict meaningful effectiveness. The FDA can require longer studies to confirm effectiveness when it gives accelerated approval. In Avastin’s case, the longer studies consistently showed adding Avastin to chemotherapy did not prolong life beyond that of the patients given chemotherapy alone. What should the FDA do now? As of this writing, the FDA decision has not been made. Here are two graphs from an original paper in the New England Journal of Medicine (1) showing the results. (Click on the image to expand it.)
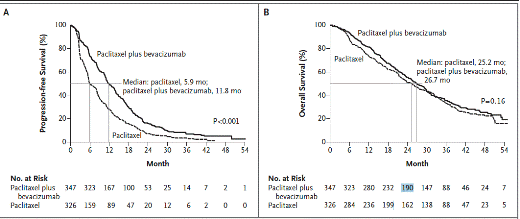
The graph on the left shows that women getting chemo plus bevacizumab (Avastin) on average took longer for the tumor to grow than those women on chemo alone. The graph on the right shows no difference in survival. About as many women on chemo alone were alive 3 or 4 years later as were women on chemo plus Avastin. This data indicates that taking Avastin did not make any difference in length of life. The women on Avastin had more infections, fatigue, nerve pain, high blood pressure, headaches, and episodes of impaired cerebrovascular blood flow than women on chemotherapy alone. Is Avastin either effective or safe for women with breast cancer? Both efficacy and safety are needed for approval.
This Avastin example is a case study of the conflict between making drugs available quicker and preventing harm due to ineffective or unsafe drugs being marketed and used. It also raises the issue of how to determine effectiveness. The new treatment must be compared to something else to learn which is better. The something else can be nothing or a placebo. Today, the usual comparison is standard care plus placebo (the control group) to either standard care plus new drug or new drug alone. How much difference should there be for a new drug to be considered effective? Is any real difference enough no matter how small the difference is? Is it sensible that cost, no matter how much, cannot enter into the FDA’s evaluation? And if the difference is marginal at best, is it enough to outweigh the known and as yet unknown harms that the new drug can produce? These are clearly judgment calls based on personal value systems. Individuals give different weights to each of the different facts including the weight they give to the degree of uncertainty about what is undiscovered at the time of review. For this reason, different people can come to different interpretations of the same facts and recommend different decisions from the same set of facts. These are the circumstances within which the FDA has to make a decision about whether to permit a drug to be marketed or not.References:
- Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007 Dec 27;357(26):2666-76.
- Miles DW, Chan A, Dirix LY, Cortés J, Pivot X, Tomczak P, Delozier T, Sohn JH, Provencher L, Puglisi F, Harbeck N, Steger GG, Schneeweiss A, Wardley AM, Chlistalla A, Romieu G. Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2010 Jul 10;28(20):3239-47.
- Robert NJ, Diéras V, Glaspy J, Brufsky AM, Bondarenko I, Lipatov ON, Perez EA, Yardley DA, Chan SY, Zhou X, Phan SC, O'Shaughnessy J. RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol. 2011 Apr 1;29(10):1252-60.
Posted 9/8/2011
This note can be found online at http://www.weill.cornell.edu/cert/patients/avastin.html
Health information for everyone from the Weill Cornell/HSS CERT http://www.weill.cornell.edu/cert/patients/